how to read ir spectra graph How to read ir spectra graph
Welcome to our post on how to read and interpret infrared (IR) spectra graphs! In this quick guide, we will provide you with all the information you need to understand these fascinating graphs and gain insights into the molecular structure of organic compounds.
How To Read IR Spectra Graph
IR spectra graphs are powerful tools used in analytical chemistry to identify functional groups and chemical bonds present in a compound. They provide valuable information about the vibrations and rotations of molecules.

Let’s break down how to read an IR spectra graph:
1. The x-axis of the graph represents the frequency or wavelength of light, usually measured in reciprocal centimeters (cm^-1) or micrometers (µm). The range is typically depicted from left to right.
2. The y-axis represents the percentage of light absorbed by the compound. The higher the peak, the more light is absorbed at a specific frequency.
3. Peaks on the graph correspond to specific functional groups or bonds. These peaks occur when molecular vibrations or rotations absorb infrared light within a certain range of frequencies. Each peak represents a different bond or group.
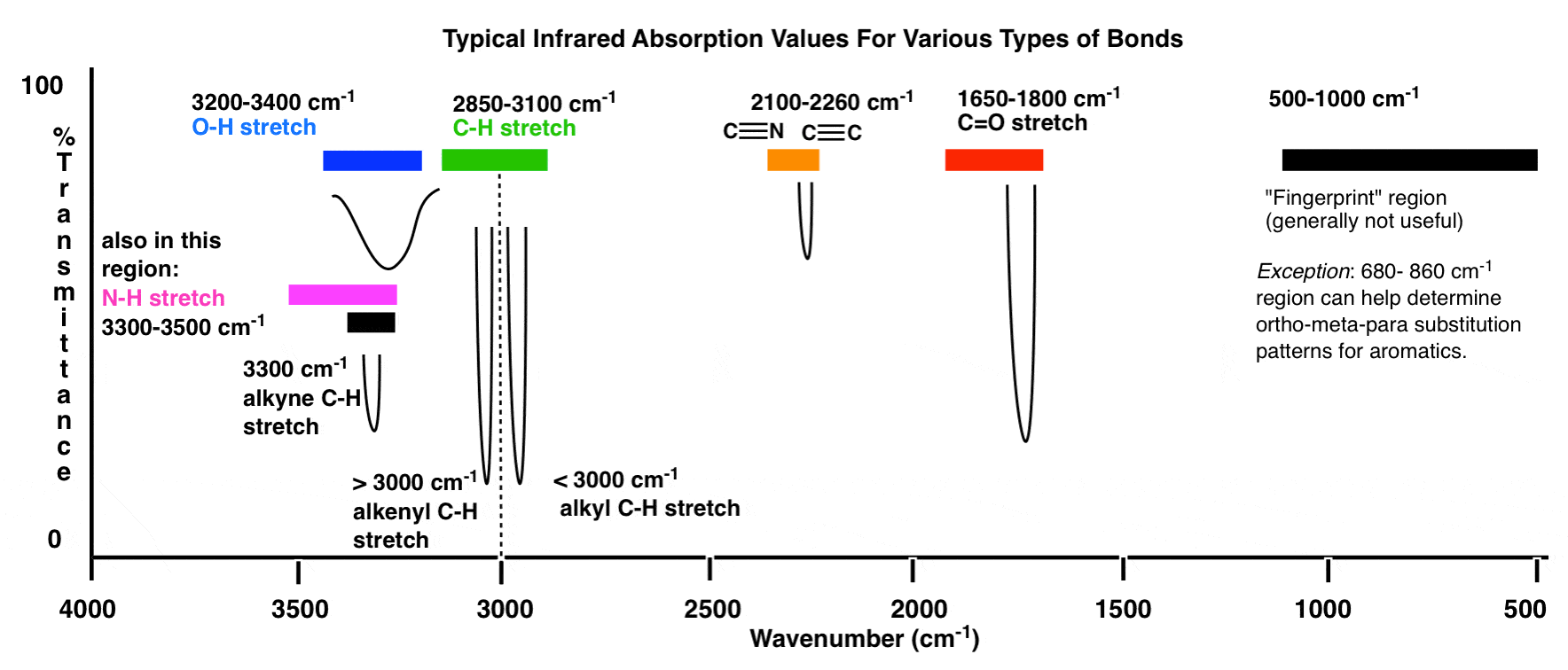
4. Different regions of the graph provide information about various types of bonds. For example, the fingerprint region (500-1500 cm^-1) contains unique patterns that can be used for compound identification.
Interpreting IR Spectra: A Quick Guide
Now that we have covered the basics of reading an IR spectra graph, let’s delve deeper into interpreting the peaks and understanding what they mean:
- Broad peaks in the 3000-4000 cm^-1 region indicate the presence of O-H (alcohols) or N-H (amines) bonds.
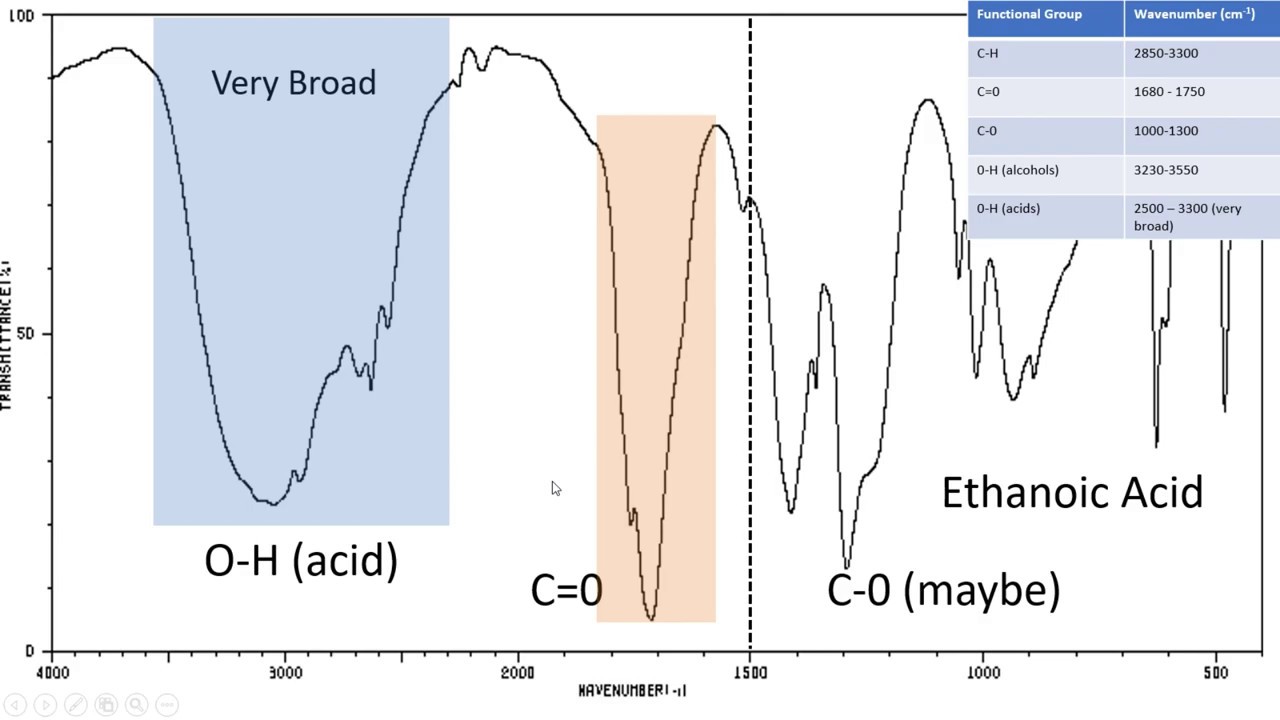
- Sharp peaks in the 1700-1800 cm^-1 region suggest the presence of C=O (carbonyl) bonds, commonly found in aldehydes, ketones, carboxylic acids, and esters.
- Peaks in the 2900-3100 cm^-1 region indicate C-H (alkane, alkene, or alkyne) bonds. The specific location on this region can provide clues about the type of bond.
- Peaks below 1500 cm^-1 indicate the presence of functional groups such as C-Cl (alkyl chloride) or C-Br (alkyl bromide) bonds.
- Fingerprints region (500-1500 cm^-1) contains unique patterns that can be used to identify specific compounds.
These are just a few examples of how to interpret peaks in an IR spectra graph. With practice and guidance, you can become proficient in interpreting these graphs and unlock a world of information about unknown organic compounds.
We hope this quick guide has provided you with a solid foundation in reading and interpreting IR spectra graphs. Remember, practice makes perfect! So, keep exploring and experimenting with different compounds to enhance your understanding. Stay curious, and the world of organic chemistry will reveal its fascinating secrets to you.
If you are looking for Interpreting IR Specta: A Quick Guide – Master Organic Chemistry you’ve visit to the right place. We have 5 Images about Interpreting IR Specta: A Quick Guide – Master Organic Chemistry like Interpretation of IR spectra, Interpreting IR Specta: A Quick Guide – Master Organic Chemistry and also How To Read Ir Spectra Graph. Here it is:
Interpreting IR Specta: A Quick Guide – Master Organic Chemistry
 www.masterorganicchemistry.comir table spectroscopy mcat spectrum spectra chart chemistry organic infrared interpreting ranges analysis guide cm absorptions regions cheat
www.masterorganicchemistry.comir table spectroscopy mcat spectrum spectra chart chemistry organic infrared interpreting ranges analysis guide cm absorptions regions cheat
How To Read Ir Spectra Graph
 www.donboscoviet.netHow To Read Ir Spectra Graph | Earth-base
www.donboscoviet.netHow To Read Ir Spectra Graph | Earth-base
 www.earth-base.orgHow To Read Ir Spectra Graph | Earth-base
www.earth-base.orgHow To Read Ir Spectra Graph | Earth-base
 www.earth-base.orgInterpretation Of IR Spectra
www.earth-base.orgInterpretation Of IR Spectra
 www.slideshare.netspectra
www.slideshare.netspectra
Ir table spectroscopy mcat spectrum spectra chart chemistry organic infrared interpreting ranges analysis guide cm absorptions regions cheat. How to read ir spectra graph. Interpreting ir specta: a quick guide – master organic chemistry